Word That Describes the Repeating Chemical Properties of Elements
In the mid-1800s Dmitri Mendeleev a Russian chemist noticed a repeating pattern of chemical properties in elements. Periodic trend - regular variation in the properties of elements with increasing atomic number.
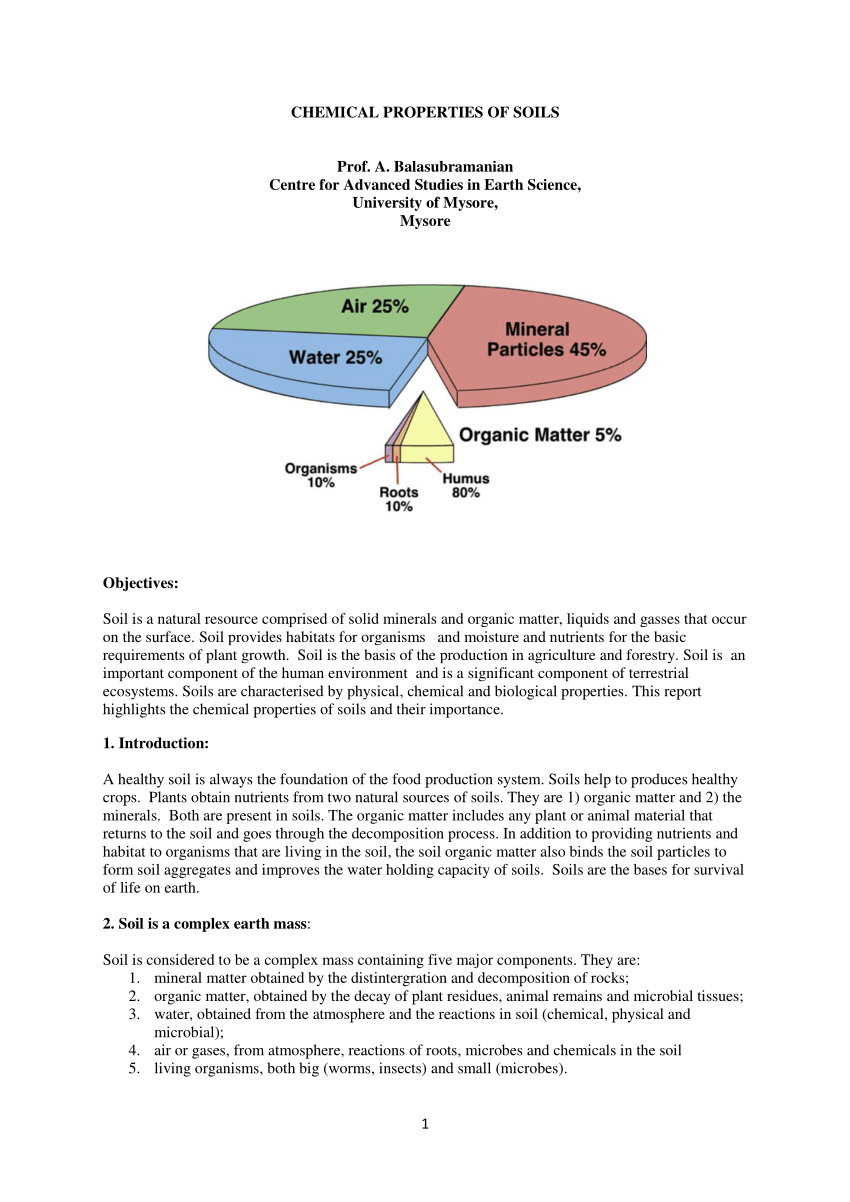
Pdf Chemical Properties Of Soils
The Periodic Table has this name because there is a repeating pattern of properties like the repeating pattern of menstrual periods.

. It is attractive in colour and brightness durable to the point of virtual indestructibility highly malleable and usually found in nature in a comparatively pure form. The ____ _____ shows the elements name atomic number chemical symbol state of matter and atomic mass. Locate the elements that are nonmetals.
A The elements are arranged in groups with similar chemical properties. During a chemical change. Gold has several qualities that have made it exceptionally valuable throughout history.
Periodic patterns are based on the Periodic Law which specifies that when chemical elements are listed according to increasing atomic number all of their properties undergo gradual changes with elements with identical properties repeating at intervals. B The elements are arranged in periods with repeating trends in properties. A chemical change is one in which the substance is transformed to a new substance.
C The elements are arranged in order of increasing atomic number. This module explains the arrangement of elements in the period table. A pattern of repeating order is called periodicity.
Other atoms are classified as nonmetals. Nonmetals can be found primarily in the upper right corner of the periodic table while the rest of. The pattern of repeating properties of elements revealed in the periodic table is known as what.
That is there is a change in the chemical composition of the substance. Because certain properties of the elements repeat on a regular basis throughout the table that is they are periodic it became known as the periodic table. Characteristics of elements that help to identify what they are.
Make you a sentence using periodic law. Matter can also be characterized by its chemical properties. The elements in the table showed repeating patterns.
Reactions with group 1 metals The halogens react with metals to produce salts the word halogen means salt former. However not all the elements fit this pattern neatly. Repeating rows of the periodic table that demonstrate patterns of how elements are arranged.
Many of the properties recur at intervals. A catalytic or thermal cracking b catalytic reforming c None of. In Mendeleevs periodic table elements were arranged on the basis of the fundamental property atomic mass and chemical properties.
During Mendeleevs work only 63 elements were known. This means that the halogens. Mendeleev arranged the elements in order of increasing atomic mass to form something that resembles the modern periodic table.
_____ is a word used to describe such patterns. Which statement about the periodic table is not correct. Previous Post Previous Based on this excerpt which word best describes Paul.
An abbreviation for the name of an element on the periodic. The periodic law is. What pattern is revealed when the elements are arranged in a periodic.
All have similar chemical properties. For his book he began to organize elements in terms of their properties by placing the elements and their newly discovered atomic masses in cards. We have explained the periodic trends for you in the paragraphs below.
Word that describes the repeating chemical properties oberts וז 12 15 1105 17. Gold Au chemical element a dense lustrous yellow precious metal of Group 11 Ib Period 6 of the periodic table. On the periodic table the majority of atoms are classified as metals.
Elements are arranged according to same chemical properties. Elements arranged in vertical columns of the periodic table that share the same properties. Nglish chemist who proposed the law of octuves another toom for the horizontal arrangement of towers in.
The repeating pattern of elements of their properties is called periodicity. The physical and chemical properties of the elements also _____ as you move from left to right across a period. The Properties of Elements Showed a Repeating Pattern At the time that atomic masses had been discovered a Russian chemist named Dimitri Mendeleev was writing a textbook.
The Periodic Law states that the physical and chemical properties of the elements recur in a systematic and predictable way when the elements are arranged in order of increasing atomic number. Periodicity - recurring variations in element properties with increasing atomic number due to trends in atomic structure. D The elements in the halogen group.
After studying the properties of every element Mendeleev found that the properties of elements were related to atomic mass in a periodic way. The chemical properties of a substance include all the chemical changes possible for that substance. The modern periodic table is based on Dmitri Mendeleevs 1896 observations that chemical elements can be grouped according to chemical properties they exhibit.
Breadth In Chemistry 2019. Metals Non - metals In the Periodic Table metals are on the left of the stepped line and non - metals are on the right Properties of metals Most metals have high melting points. When the elements were ordered according to atomic weight Mendeleev like de Chancourtois and Newlands could see that certain chemical properties were repeated periodically.
You will find these groupings beneficial as you explore the properties of different atoms. It defines periods and groups and describes how various electron configurations affect the properties of the atom. Mendeleev had to list some elements out of the order of their atomic masses to group them with other elements that had similar properties.
Periodicity or periodic law. Whoa there Wind I said softly as I tried to get Whoa there Wind I said softly as I tried to get Next Post Next The conversion of large hydrocarbon molecules into smaller molecules is known as. 18 10 sorchet saw that means liest pirst word of the colleclivn nemo for the elements in cirup ba russian cumist who proposed the arrangement of the banimingai.

Activity 14 Physical And Chemical Properties Of Materials Ppt Download
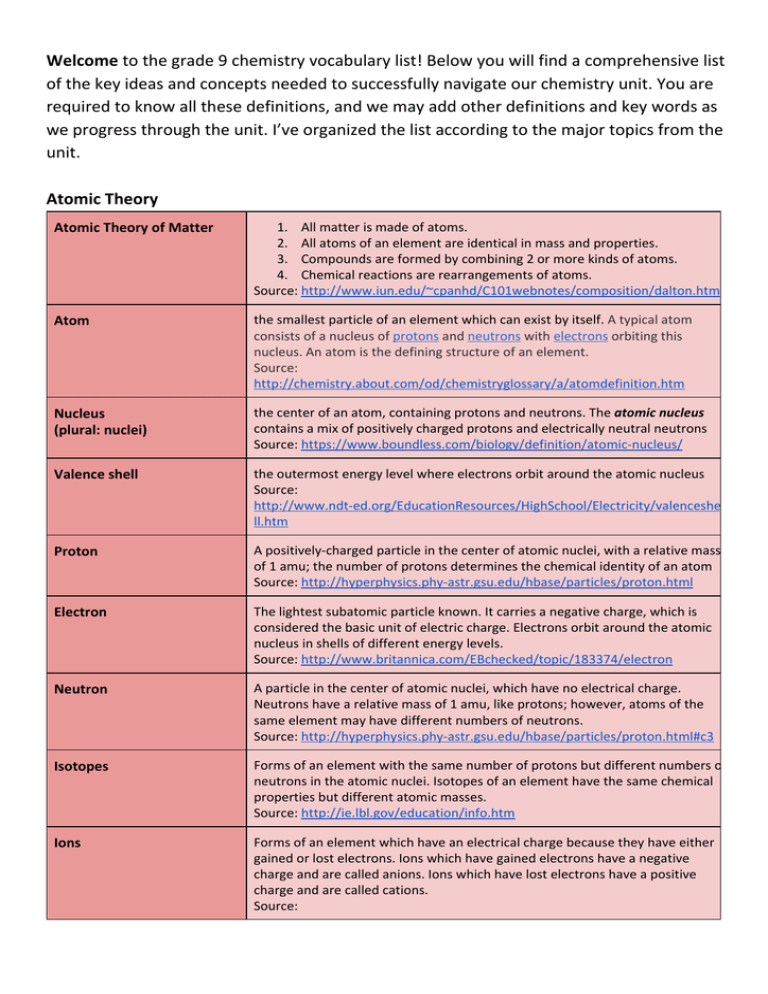
The Grade 9 Chemistry Vocabulary List
Electron Configuration And Periodicity Prezentaciya Onlajn

Cbse Class 10 Chemistry Differentiate Between Metal And Non Metal On The Basis Of Their Chemical Properties

Proteins Fundamental Chemical Properties

Periodic Table And Chemical Properties Periodic Table Chemical Property Periodic Table Of The Elements

Atom And Elements Vocabulary Scavenger Hunt Vocabulary Formative Assessment Vocabulary Words

Pdf Biodegradation Of Nonlignocellulosic Substances Ii Physical And Chemical Properties Of Sawdust Before And After Use As Artificial Soil

Periodic Law The Periodic Law States That Physical And Chemical Properties Of The Elements Are Periodic Functions Of Their Atomic Numbers In Other Words Ppt Download

Periodic Table It Is A Systematic Catalog Of The Elements Ppt Video Online Download

Atomic Structure The Periodic Table Ppt Download

Synthesis Physical Vs Chemical Properties Changes Physical Vs Chemical Properties Physics Teaching Chemistry

Activity 14 Physical And Chemical Properties Of Materials Ppt Download

I Always Copy Worksheets Homework And Take Notes By Doing This You Can Be More Prepared I Also Record Myself Stating The Science Notes Read Aloud Teaching

Chapter 12 Material On Midterm Ppt Download

Metals And Their Properties Metals Have Distinctive Properties Such As 1 Electrical Conductivity 2 Good Therm Gcse Science Teacher Notes Class Participation



Comments
Post a Comment